What is Spinal Fusion
Spinal fusion may be a solution for some patients with degenerative disc disease who are not candidates for total disc replacement—providing the stability and security needed to help the spine heal.
Spinal fusion may be a solution for some patients with degenerative disc disease who are not candidates for total disc replacement—providing the stability and security needed to help the spine heal.
Spinal fusion is a surgical procedure used to fuse together two or more vertebrae, so they heal into a single bone. For patients with back problems such as degenerative disc disease, fractured vertebra or a herniated disk, the spinal fusion treatment can create invaluable security and eliminate ongoing back pain.
Learn More About the Benefits of Fusion Surgery from the Front Versus the Back
Watch the Video
STALIF is an Integrated Interbody fusion platform that offers a surgical treatment proven to restore spinal balance, recreate spinal alignment and stability, and accelerate the return to normal activities.
Centinel Spine was the first company in the world to offer Integrated Interbody (a.k.a. Stand-Alone) fusion devices, and has a long clinical history in providing fusion solutions that conform to patient anatomy and capitalize on the body’s ability to heal itself.
Available devices include:
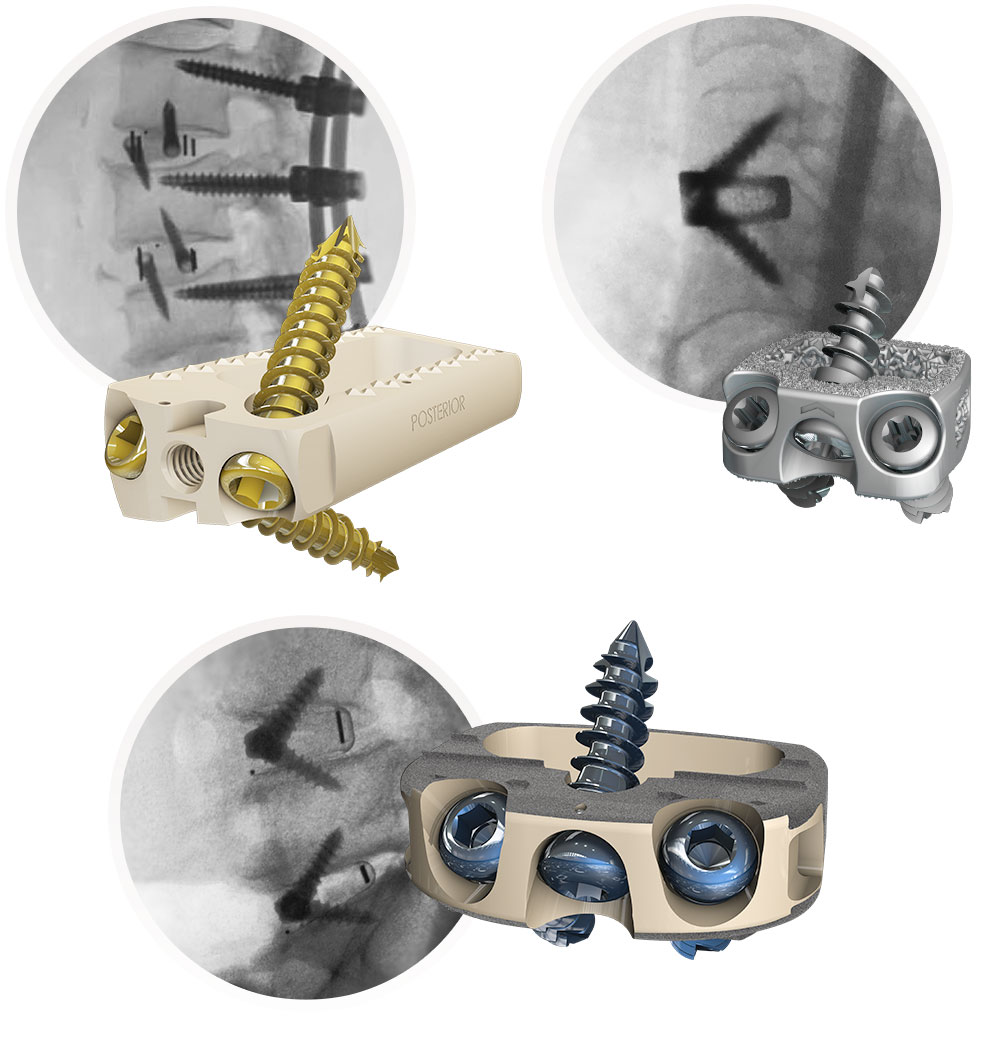
The 1st implantation of a STALIF device took place in 1988. Since the very first device was implanted until now, Centinel Spine has continued to innovate and improve upon its original STALIF design, with state-of-the-art solutions now existing in multiple material options and device sizes to best suit each patient’s unique needs.
The STALIF line of Integrated Interbody fusion implants are the longest implanted line of Integrated Interbody devices in the world.
30+
30+ Year Clinical History with Worldwide Usage
Unparalleled clinical & biomechanical data, with average fusion rates of 92-95% 1,2,3
More than 75,000 device implantations and a reported reoperation rate of less than 1%
STALIF technology incorporates a proven design rationale based on AO principles of fracture fixation and Wolff’s Law of Bone Healing.
Wolff’s Law of Bone Healing states that bone grows where load is applied. That is why STALIF devices utilize specially designed screws that pull the spinal bones at the fusion site down to the device, in what is known as a "lag effect"—providing an active load to aid in the healing process.
Above: The Lag Effect is demonstrated.
STALIF devices are also designed to conform to patient anatomy. They come in a variety of sizes, heights, angles, and materials to fit each patient’s needs and offer the best chance of successful outcomes.
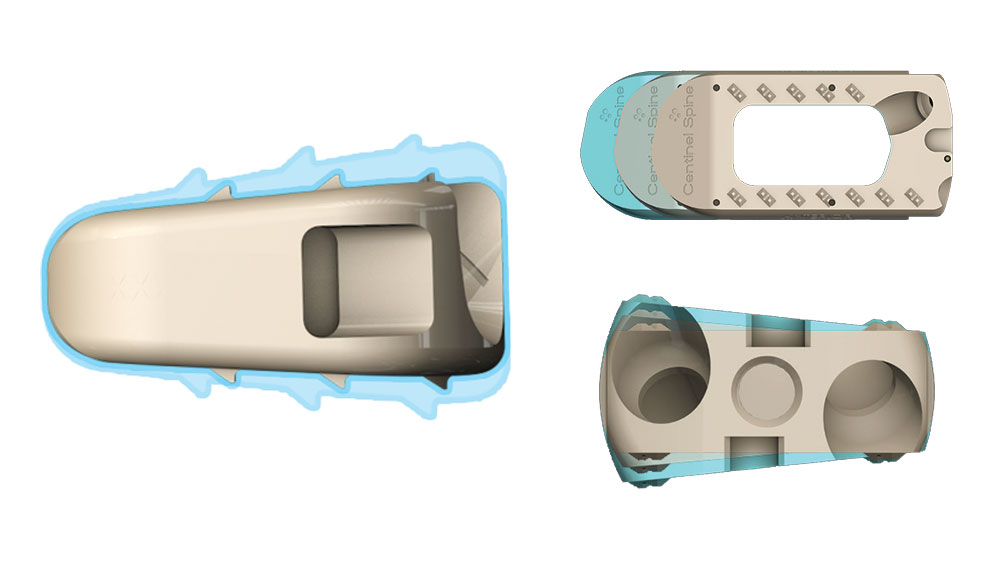
STALIF was developed with several specific patient care objectives in mind:
STALIF devices are designed to conform to patient anatomy. They come in a variety of sizes, heights, and angles to fit each patient’s needs, so that spinal curvature, height, and alignment can be restored, offering the best chance for successful outcomes.
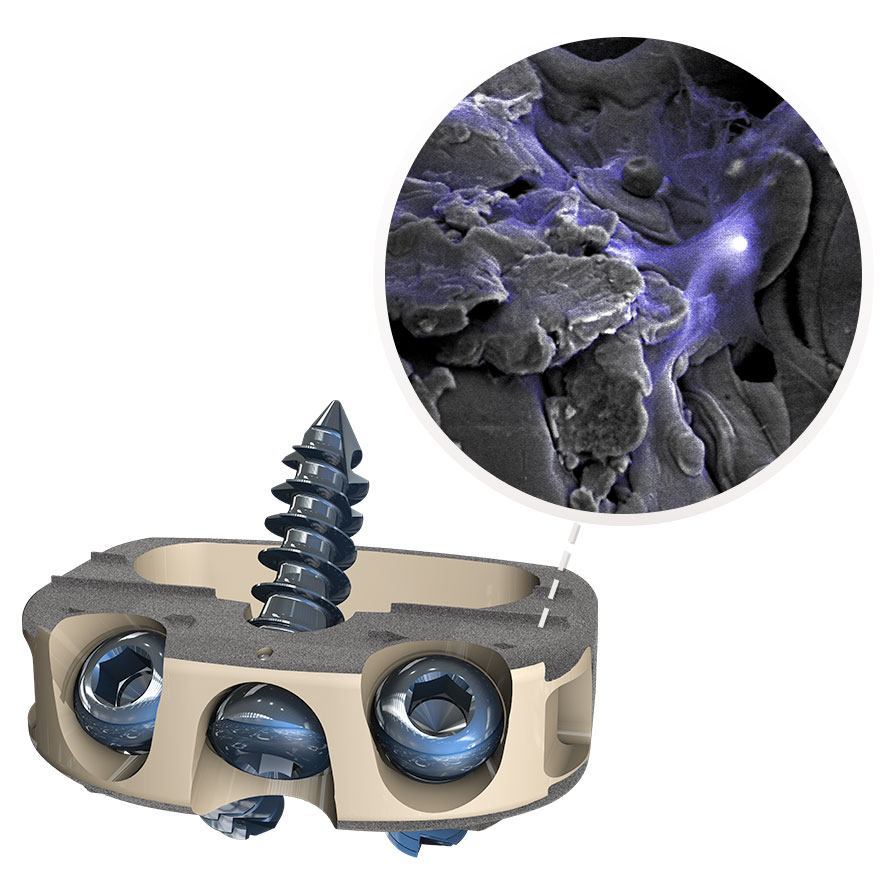
STALIF devices come in a variety of material options to enhance opportunities for fusion based on patient specific needs. Some STALIF implants incorporate materials, such as titanium, that are known to be cell-friendly and attract bone cells. They also have very large cavities that allow for a large amount of bone to form between the vertebral bodies to make a solid fusion. Combining these features with the compressive design and the ability to pick an implant that fits each patient’s anatomy optimally enhances opportunities for successful fusion outcomes.
Degenerative disc disease can be very painful, and recovery from spinal surgery can take time. Special care has been taken to design STALIF devices to minimize the recovery associated with the procedure. STALIF devices are implanted from the front (anterior) of the body, going in through either the abdomen (ALIF), side (LLIF), or throat (ACDF), so stabilizing spinal muscles and bony elements are not disturbed. Studies have shown that compared to more invasive fusion procedures that approach the spine by going in through the back (posterior), patients who receive fusions from anterior approaches have shorter hospital stays (1-3 days vs. 3-6 days), mobilize more quickly (many patients are walking the same day they have surgery), and lose much less blood during the procedure.
STALIF devices are also designed to be fully contained within the anatomy of the spinal bones, so the risks of post-operative complications associated with other body anatomy, such as difficulty swallowing (dysphagia), are minimized.

With a clinical heritage of more than 30 years, STALIF has been extensively studied.
After multiple published studies, the clinical & biomechanical data for STALIF strongly supports its safety and effectiveness. Here are just a few key takeaways from these numerous studies:
Better than or comparable to reported rates using traditional plate & cage constructs 1,2,3
Lower instances of adjacent segment degeneration and an 85% reduction in reported instances of dysphagia vs traditional plate & cage constructs 4
No Profile STALIF devices are a safe alternative to rigid anterior plating for 1 or 2 level cervical fusion procedures and STALIF lumbar devices with 3-screw fixation provide equivalent motion restriction to a construct with an anterior non-integrated cage & posterior pedicle screw fixation
For more information about STALIF choose one of the below options.
Print the STALIF Info Card for Your Doctor's Visit
DownloadView the STALIF M/L Patient Guide
DownloadView the STALIF C Patient Guide
DownloadHear from a Patient Ambassador
Get Inspired →
STALIF technology may not be suitable for all cervical fusion patients. In some situations, the use of a non-integrated interbody device may be needed. The ACTILIF C Portfolio of non-integrated interbody devices is proven to be a viable option.
ACTILIF C is a non-integrated anterior fusion device for the upper (cervical) spine (C2-T1) that is used in conjunction with a supplemental fixation option, such as a cervical plate, available in 2 material options (PEEK and FLX).

WORKING WITH your insurer
While the vast majority of insurance plans cover STALIF and ACTILIF, some fusion devices may be considered investigational by your insurance provider. Though reimbursement coverage for stand-alone fusions continues to increase, not all insurance companies cover these procedures. Centinel Spine offers a Patient Assistance Line (PAL) to qualified patients to help them to navigate insurance coverage for this surgery.
If you are not covered by your insurance provider, the PAL (Patient Assistance Line) is available to help you gain coverage for STALIF or ACTILIF procedures. The PAL is a reimbursement support service that assists YOU, the patient. Your PAL is dedicated to assisting YOU and working together with your surgeon, medical office, and insurance provider.
Important Patient Information: The resources provided on this site are for informational purposes only.
This website is not a replacement for professional medical advice. You should discuss both surgical and nonsurgical treatment options with your doctor.
Only your doctor is qualified to diagnose and treat your condition.
Problems can occur when you have spine surgery, including surgery with STALIF or ACTILIF implants. There is a risk that the surgery may not make you feel better or
may cause you to feel worse. If this happens, you may need another surgery to help you feel better. View the specific problems that can occur during or
after STALIF M, STALIF C, STALIF L, or ACTILIF C surgery.
Reimbursement is dynamic. Coding, coverage and payment are subject to change. Centinel Spine cannot guarantee reimbursement for any procedure associated
with the use of its products. Providers should contact their specific payers if they have questions regarding coding, coverage or payment.
1 Elshihabi, Said, “A Retrospective Clinical and Fusion Analysis of the Device with Stem-Cell Derivative Bone Graft Material”, Presented at The Southern Neurosurgical Society’s 66th Annual Meeting, Naples, FL., March 2015.
2 Lane, Paul, et al., “Early Radiographic and Clinical Outcomes Study Evaluating an Integrated Screw and Interbody Spacer for One- and Two-Level ACDF”, The International Journal of Spine Surgery, 9(39). CI: 10.14444/2039.2015.
3 Vokshoor, Amir, et al., “Retrospective Clinical Outcome Study Evaluating the Efficacy of the Stand-Alone Cervical Device in Anterior Cervical Discectomy and Fusion”, Presented at The ISASS 15th Annuas Conference, San Diego, CA., April 2015.
4From STALIF C Clinical Compendium, Section 6
These individuals can inspire your journey to healing and life after disc replacement surgery—and the hope of freedom from spine-induced pain and discomfort.
To Find a Spine Surgeon That's Right for You, Use our Surgeon Locator